ORYZON reports results and corporate update for Q4 and year ended December 31, 2021
- The company allocated $1.5 million in legal and regulatory preparations to adequate the company for a future Nasdaq dual listing
MADRID, SPAIN and CAMBRIDGE, MA, UNITED STATES, February 7th, 2022 – Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company leveraging epigenetics to develop therapies in diseases with strong unmet medical need, today reported financial results for the fourth quarter of 2021 and provided an update on recent developments.
Dr Carlos Buesa, Oryzon’s Chief Executive Officer, said: “We made strong progress on our clinical pipeline in 2021. In oncology, we completed the full enrollment of patients in iadademstat’s Phase II trial in acute myeloid leukemia (AML) and presented new positive clinical data from this trial, most recently at ASH in December, supporting the strong clinical activity of iadademstat. Also, we have unfolded our registrational strategy in AML and in Extensive-Disease Small Cell Lung Cancer (ED-SCLC) with two new carefully designed trials to start in 2022, FRIDA and STELLAR, that we believe may merit accelerated approval.”
“In CNS, we also reached important milestones. Vafidemstat’s Phase IIb PORTICO in BPD concluded its deployment phase, we received IND approval from the FDA in June and we are now actively recruiting patients in the USA, Spain, Germany, Bulgaria and Serbia. We have also obtained approval and initiated recruitment for our vafidemstat’s Phase IIb in schizophrenia, called EVOLUTION. Also, together with the most prestigious KOLs in the space, we are progressing the design of HOPE, the first randomized Phase I/II personalized medicine trial with an LSD1 inhibitor, in particular in Kabuki Syndrome patients, which we expect to start in the first half of 2022. Oryzon has further strengthened its permanent presence in the US with the appointment of two new US-based executives: we welcome Sai Nandi as our Global Chief Business Officer to optimize US investor relations and corporate relations, and Ana Limón as Senior VP of Clinical Development and Global Medical Affairs to coordinate the US clinical activities and portfolio deployment. We finished this fourth quarter with a solid cash position of $32.5 million, which provides funding for further development of our exciting pipeline until Q1 2023.”
Fourth Quarter and Recent Highlights
Iadademstat in oncology:
- The Phase II ALICE trial, investigating iadademstat in combination with azacitidine in acute myeloid leukemia (AML), is fully enrolled, with a total of 36 patients. Preliminary data corresponding to the 36 months of the study were presented at the ASH 2021 congress last December, showing robust signs of clinical efficacy, with ORR of 78%, of which 62% were CR/CRi, as well as a good safety profile for the combination of iadademstat and azacitidine. The duration of observed responses was very encouraging, with 77% of the CR/CRi lasting over 6 months. The longest remission at the data cut-off date for ASH-2021 was over 1,000 days, and is still ongoing, with the patient remaining transfusion independent and MRD-negative. The company plans to present a new clinical update on ALICE at the EHA-2022 congress and final data at ASH-2022.
- New trials in combination in AML and solid tumors are under preparation. In AML, the company is planning to launch FRIDA, an open-label, multicenter Phase Ib/II trial of iadademstat in combination with gilteritinib in FLT3 mutated relapsed/refractory AML patients. In small cell lung cancer (SCLC), the STELLAR trial is in preparation. STELLAR is a randomized, multicenter Phase Ib/II study of iadademstat plus a checkpoint inhibitor in first line extensive disease SCLC. Both trials will be conducted in the US and the plan for each is to enroll 120 patients. The company believes that FRIDA and STELLAR could potentially support applications for accelerated approval.
- Oryzon published a scientific paper in the peer-reviewed international scientific journal, ACS Pharmacology & Translational Science supporting best-in-class performance of iadademstat in oncology. The article reports a comprehensive comparison of iadademstat with most of the LSD1 inhibitors in development and shows that iadademstat is consistently the most active compound across diverse cancer cell lines, that its capability to bind the target is superior, specially at low concentrations, and that the disruption of the transcriptional complexes implicated in the oncogenic programs is more efficacious in the case of iadademstat.
Vafidemstat in large multifactorial CNS indications:
- Received approval for the Serbian arm of the PORTICO Phase IIb clinical trial with vafidemstat in patients with Borderline Personality Disorder (BPD), completing the deployment phase of this trial. The trial is now actively recruiting patients in Europe and the US. PORTICO is a multicenter, double-blind, randomized, placebo-controlled Phase IIb to evaluate the efficacy and safety of vafidemstat in BPD patients. The trial has two independent primary objectives: reduction of aggression/agitation and overall BPD improvement. The study will include 156 patients, with 78 patients in each arm, and has a pre-defined interim analysis to adjust the sample size in case of excessive variability around the endpoints or an unexpectedly high placebo rate. The trial will be conducted in 15-20 sites in Europe and US.
- Enrollment of the first patient in the EVOLUTION Phase IIb clinical trial with vafidemstat in patients with schizophrenia. This Phase IIb study aims to evaluate the efficacy of vafidemstat on negative symptoms and cognitive impairment in patients with schizophrenia. This project is partially financed with public funds from the Spanish Ministry of Science and Innovation and will be carried out in various Spanish hospitals. We have activated various clinical sites in the period.
Vafidemstat in monogenic CNS indications:
- We have continued to made progress in the preparation of a new precision medicine trial in Kabuki syndrome (KS) patients. This Phase I/II trial, named HOPE, will be a multicenter, multi-arm, randomized, double-blind and placebo-controlled trial to explore the safety and efficacy of vafidemstat in improving several impairments described in KS patients. The trial plans to enroll 50-60 patients and will be performed in children older than 12 years and in young adults. The company expects to start HOPE in the first half of 2022 in several hospitals and sites in the United States and, possibly, in Europe. Considering the FDA and EMA precedents in rare diseases and CNS disorders, we believe that if the HOPE trial demonstrates relevant clinical improvements, it may potentially serve as the basis for accelerated approval in the EU and the United States.
- Our precision medicine programs in psychiatric disease continue to progress. We have collaborations in autism with researchers at the Seaver Autism Center for Research and Treatment at Icahn School of Medicine at Mount Sinai Hospital in New York and the Institute of Medical and Molecular Genetics (INGEMM) at Hospital Universitario La Paz of Madrid and in schizophrenia with researchers from Columbia University in New York. The results of the ongoing pilot studies to characterize patients with specific mutations to inform subsequent precision psychiatry clinical trials with vafidemstat are expected to conclude in 2022.
Financial Update: Fourth Quarter 2021 Financial Results
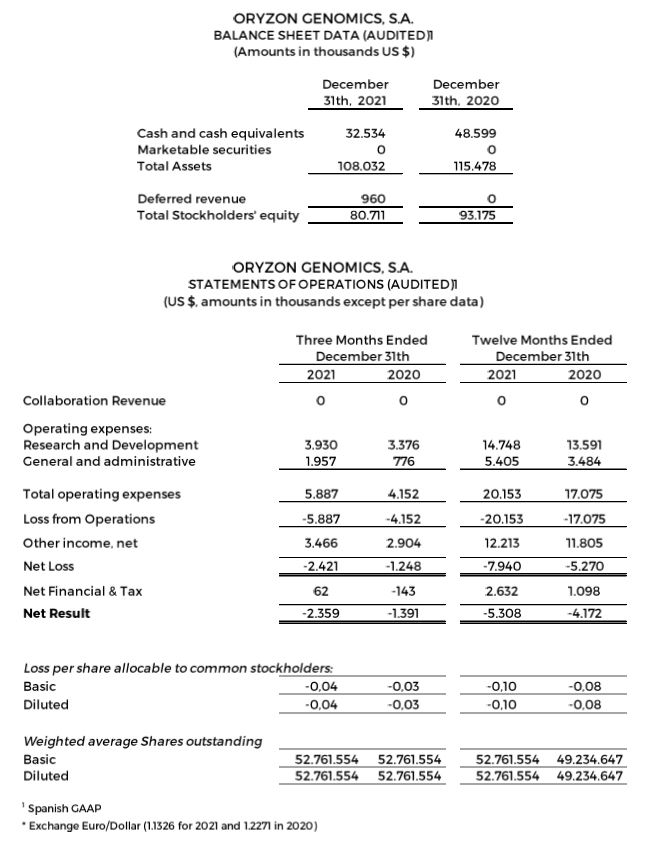
Research and development (R&D) expenses were $3.9 and $14.8 million for the quarter and 12 months ended December 31, 2021, compared to $3.4 and $13.6 million for the quarter and 12 months ended December 31, 2020.
General and administrative expenses were $2.0 and $5.4 million for the quarter and 12 months ended December 31, 2021, compared to $0.8 and $3.5 million for the quarter and 12 months ended December 31, 2020.
Net losses were $2.4 and $7.9 million for the quarter and 12 months ended December 31, 2021, compared to net losses of $1.2 and $5.3 million for the quarter and 12 months ended December 31, 2020. This is due to a higher investment in research and non-capitalized development of the ESCAPE clinical trial and non-recurring expenses, including $1.5 million in legal and regulatory preparations to adequate the company for a future Nasdaq dual listing. The result is in accordance with the specificity of the biotechnology business model, in the development phase of the Company, with a long-term maturation period for its products, and without recurrent income.
Negative net result of $5.3 million (-$0.10 per share) for the 12 months ended December 31, 2021, compared to a negative net result of $4.2 million (- $0,08 per share) for the 12 months ended December 31, 2020.
Cash, cash equivalents and marketable securities totaled $32.5 million as of December 31, 2021, compared to $48.6 million as of December 31, 2020.
During the year, Oryzon has obtained a grant of $1 million to support a new clinical trial (the HOPE trial) in patients with Kabuki syndrome.
About Oryzon
Founded in 2000 in Barcelona, Spain, Oryzon (ISIN Code: ES0167733015) is a clinical stage biopharmaceutical company considered as the European champion in Epigenetics. Oryzon has one of the strongest portfolios in the field. Oryzon’s LSD1 program has rendered two compounds, vafidemstat and iadademstat, in Phase II clinical trials. In addition, Oryzon has ongoing programs for developing inhibitors against other epigenetic targets. Oryzon has a strong technological platform for biomarker identification and performs biomarker and target validation for a variety of malignant and neurological diseases. Oryzon has offices in Spain and the United States. Oryzon is one of the most liquid biotech stocks in Europe with +90 M shares negotiated in 2020 (ORY:SM / ORY.MC / ORYZF US OTC mkt). For more information, visit www.oryzon.com
About Iadademstat
Iadademstat (ORY-1001) is a small oral molecule, which acts as a highly selective inhibitor of the epigenetic enzyme LSD1 and has a powerful differentiating effect in hematologic cancers (see Maes et al., Cancer Cell 2018 Mar 12; 33 (3): 495-511.e12.doi: 10.1016 / j.ccell.2018.02.002.). A FiM Phase I/IIa clinical trial with iadademstat in R/R AML patients demonstrated the safety and good tolerability of the drug and preliminary signs of antileukemic activity, including a CRi (see Salamero et al, J Clin Oncol, 2020, 38(36): 4260-4273. doi: 10.1200/JCO.19.03250). In a still ongoing Phase IIa trial in elder 1L-AML patients (ALICE trial), iadademstat has shown encouraging safety and efficacy data in combination with azacitidine. Beyond hematological cancers, the inhibition of LSD1 has been proposed as a valid therapeutic approach in some solid tumors such as small cell lung cancer (SCLC), neuroendocrine tumors, medulloblastoma and others. In a Phase IIa trial in combination with platinum/etoposide in second line ED-SCLC patients (CLEPSIDRA trial, finalized), preliminary efficacy results have been reported. In total iadademstat has been tested in four clinical trials in more than 100 patients.
About Vafidemstat
Vafidemstat (ORY-2001) is an oral, CNS optimized LSD1 inhibitor. The molecule acts on several levels: it reduces cognitive impairment, including memory loss and neuroinflammation, and at the same time has neuroprotective effects. In animal studies vafidemstat not only restores memory but reduces the exacerbated aggressiveness of SAMP8 mice, a model for accelerated aging and Alzheimer’s disease (AD), to normal levels and also reduces social avoidance and enhances sociability in murine models. In addition, vafidemstat exhibits fast, strong and durable efficacy in several preclinical models of multiple sclerosis (MS). Oryzon has performed two Phase IIa clinical trials in aggressiveness in patients with different psychiatric disorders (REIMAGINE) and in aggressive/agitated patients with moderate or severe AD (REIMAGINE-AD), with positive clinical results reported in both. Additional finalized Phase IIa clinical trials with vafidemstat include the ETHERAL trial in patients with Mild to Moderate AD, where a significant reduction of the inflammatory biomarker YKL40 has been observed after 6 and 12 months of treatment, and the pilot, small scale SATEEN trial in Relapse-Remitting and Secondary Progressive MS, where antiinflammatory activity has also been observed. Vafidemstat has also been tested in a Phase II in severe Covid-19 patients (ESCAPE) assessing the capability of the drug to prevent ARDS, one of the most severe complications of the viral infection, where it showed significant anti-inflammatory effects in severe Covid-19 patients. Currently, vafidemstat is in two Phase IIb trials in borderline personality disorder (PORTICO) and in schizophrenia patients (EVOLUTION). The company is also deploying a CNS precision medicine approach with vafidemstat in genetically-defined patient subpopulations of certain CNS disorders.
FORWARD-LOOKING STATEMENTS
This communication contains, or may contain, forward-looking information and statements about Oryzon, including financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, capital expenditures, synergies, products and services, and statements regarding future performance. Forward-looking statements are statements that are not historical facts and are generally identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although Oryzon believes that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Oryzon shares are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Oryzon that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the documents sent by Oryzon to the Spanish Comisión Nacional del Mercado de Valores (CNMV), which are accessible to the public. Forward-looking statements are not guarantees of future performance and have not been reviewed by the auditors of Oryzon. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date they were made. All subsequent oral or written forward-looking statements attributable to Oryzon or any of its members, directors, officers, employees or any persons acting on its behalf are expressly qualified in their entirety by the cautionary statement above. All forward-looking statements included herein are based on information available to Oryzon on the date hereof. Except as required by applicable law, Oryzon does not undertake any obligation to publicly update or revise any forward‐looking statements, whether as a result of new information, future events or otherwise. This press release is not an offer of securities for sale in the United States or any other jurisdiction. Oryzon’s securities may not be offered or sold in the United States absent registration or an exemption from registration. Any public offering of Oryzon’s securities to be made in the United States will be made by means of a prospectus that may be obtained from Oryzon or the selling security holder, as applicable, that will contain detailed information about Oryzon and management, as well as financial statements.